By Centers for Disease Control and Prevention - Centers for Disease Control and Prevention, Public Domain, https://commons.wikimedia.org/w/index.php?curid=30509337
Summary - Vitamin U may play a supportive role in correct neural tube formation, a process that depends on the methylation of dUMP to form dTMP via the folate cycle. The folate and methionine cycles work together to meet the methylation needs of the body. Low cellular methylation status diverts methyl groups from the folate cycle to the methionine cycle, thereby decreasing dTMP synthesis and increasing the risk of neural tube defects. Dietary methyl donors that enter the methionine cycle directly (e.g. methionine, betaine, Vitamin U) support neural tube formation by reducing this methyl group drain. While folate is the most important factor affecting neural tube formation, folate intake may not be sufficient in and of itself due to other factors such as polymorphisms within these two pathways and intake of other components that contribute to methylation homeostasis. Talk to your dietitian before conception about designing a diet that reduces the risk of neural tube defects in your baby.
Neural tube defects (NTDs) are a collection of medical conditions in which the neural tube of the baby does not form completely during early development, exposing the spinal cord and/or brain with permanent disability resulting. Two common neural tube defects are spina bifida (spine) and anencephaly (brain).
What causes NTDs? Like many conditions, NTDs result from a combination of environmental and genetic factors. The most common environment factor is the insufficient intake of folate by the mother just before and during the first few weeks of pregnancy. Folate is a vitamin (B9), and is therefore an essential component of one's diet. In the 1950s it was noted that pregnant women taking anti-folate drugs to treat cancer gave birth to babies afflicted with congenital abnormalities like NTDs (Safi 2012)
. It was established that folate is essential for embryonic development, that women whose folate levels were low were at greater risk of having babies with NTDs, and that supplementation by the mother-to-be with folate substantially reduced the risk of NTD occurrence (Smithells 1980;
Wald 1991). Folate supplementation is the most effective way to prevent NTDs, reducing risk by 50-70%. Consequently, medical authorities recommend young women ensure their diet contains 400 ug of folate per day through food and supplements in case they become pregnant.
How does folate prevent neural tube defects? The function of folate is to transfer methyl groups generated from serine to a range of molecules throughout the cell. In embryos, these methyl groups are essential to make nucleotides required for DNA synthesis. There are many compounds that are methylated as a result of the action of folate. However, there is one for which there is evidence that a shortage of results in neural tube defects - dTMP (deoxythymidylate). There are four nucleotides used to make DNA - abbreviated to A, T, C, G. dTMP is a precursor in the formation of dTTP, usually shortened to T. In humans, the nucleotide dTMP is made from dUMP by the transfer of a methyl group from folate. Embryos supplemented with dTTP do not develop neural tube defects despite very low folate levels, indicating that dTMP shortage is the causal factor (Leung 2013). At conception, nucleotides must be synthesized in utero, and therefore a shortage of folate results in low production of dTMP, which in turn results in NTDs.
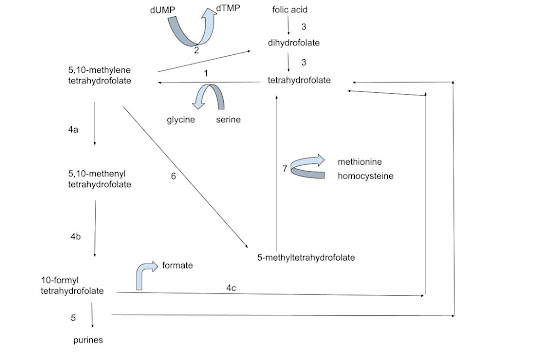
Figure 1 - A simplified depiction of the folate cycle. The enzymes responsible for catalyzing each step are -
1. Serine hydroxymethyltransferase
2. Thymidylate synthase
3. Dihydrofolate reductase
4a. 5,10-methylenetetrahydrofolate dehydrogenase NADP+
4b. 5,10-methenyltetrahydrofolate cyclohydrolase
4c. Formate-tetrahydrofolate ligase
5. Phosphoribosylaminoimidazolecarboxamide formyltransferase
6. Methylenetetrahydrofolate reductase
7. Methionine synthase
N.B. 4a-c are three components of MTHFD1
Which folate should you take and where should you get it from?
First a note on nomenclature. The term 'folate' is commonly used to refer to any of the components of this pathway plus other forms like folic acid and folinic acid. Naturally-occurring folate is a mixture of these forms, with the exception of folic acid, which is a non-natural, oxidized version that is relatively stable and therefore used to fortify food in which natural folate has been removed or degraded. Vitamin supplements usually contain either folic acid or 'methyl folate' or 'activated folate', which usually refers to 5-methyltetrahydrofolate.
The best source of natural folates are green leafy vegetables, although all vegetable sources (including fruit and grains) are reasonable sources. Folic acid is a form of folate that is often added to processed grains that have been polished, e.g. wheat flour, white rice. For most people, it makes little difference whether their folate is derived from natural or synthetic sources. The important factor is getting the right amount. However, some people do not metabolize synthetic folic acid effectively and as such can actually suffer from a deficiency in functional folate even when their serum levels of folic acid seem sufficient (Bailey and Ayling 2009). Sometimes too much folic acid can even cause fertility problems (Cornet 2019).
There are other components in the folate cycle that also have an effect on neural tube formation, albeit to a lesser extent. For example, vitamin B12 is an essential cofactor for the enzymatic conversion of 5-MTHF to THF, and low B12 levels have been linked to NTDs (Li 2009). Another example is Vitamin B6, which is a coenzyme for serine hydroxymethylfolate transferase. Cobalt is a component of B12 and therefore is vital for methionine synthase function.
Where does genetics fit in? While folate supplementation reduces risk, unfortunately genetic polymorphisms in the mother are responsible for the majority of NTDs cases (Copp 2013). Mutations of genes in the folate cycle and other branches of one carbon metabolism (methylation) are particularly relevant. There are many enzymatic steps in the folate cycle, and each enzyme is encoded by a gene. Polymorphisms are nucleotide changes (mutations) in these genes that differ from that found in the majority of people. Most polymorphisms have no significant physiological effect by themselves, but may have an effect in combination. However, there are a few polymorphisms that do correlate with the occurrence of NTDs, such as MTHFR C677T and MTHFD1 R653Q (Copp 2013).
Genetic testing is becoming an increasing-popular tool to identify polymorphisms. Though it is tempting to do so, it is important to not assume that polymorphisms necessarily result in reduced physiological function. Human physiology is not fully understood and often contains redundancies that can mask over minor metabolic blocks. The correct way to establish whether there are deficiencies within the folate cycle is through biochemical analysis of the various folate metabolites. Biochemical analysis in combination with genetic analysis is used by specialists to establish whether there is a functional deficit in the mother that results in heightened risk of NTDs in her baby. Consult with your doctor or dietitian to determine if you have polymorphisms, and if you do, whether these actually affect your metabolism (often they don't) and what measures can be taken to relieve any metabolic blocks.
Aside from the folate cycle, there is another cycle that is even more important in meeting our methylation needs. The methionine cycle is the way in which the methyl donor S-adenosylmethionine (SAM) is generated (
methionine cycle). SAM is the methyl donor for just about all methylation reactions, with the notable exception of those in the folate cycle. It has been shown that a functioning methionine cycle is essential for correct neural tube formation (
Leung 2017).
When there is a shortage of methyl groups in the methionine cycle (low S-adenosylmethione:S-adenosylhomocysteine), methyl groups are directed from the folate cycle into the methionine cycle. Instead of being used to make nucleotides, 5,10-methylenetetrahydrofolate is reduced by MTHFR to make 5-methyltetrahydrofolate, which donates its methyl group to the methionine cycle in a reaction catalyzed by methionine synthase. The folate molecule is conserved within the folate cycle, but must be remethylated. When maternal folate levels are low and flux through the folate cycle is already slow, this shunt may reduce methylation capacity in the folate cycle to critical levels.
The methionine cycle obtains a large amount of its methyl groups from the folate cycle. This shunt operates at moderate levels most of the time - this is actually a normal process. In addition, the methionine cycle obtains methyl groups from methionine, betaine and Vitamin U. Similar to the methionine synthase reaction, betaine and Vitamin U donate methyl groups to homocysteine via enzyme-catalyzed reactions.
Importantly, methyl groups in the methionine cycle cannot enter the folate cycle. For example, SAM from the methionine cycle cannot substitute for 5,10-MeTHF required for
dTMP synthesis. Low maternal methionine levels pre- and post-conception are associated with a heightened risk of neural tube defects (
Shaw 2004).
There is evidence that betaine entering the methionine cycle reduces the flow of methyl groups from the folate cycle, which in principle should support 5,10-MeTHF levels (Benevenga 2007)
.Where does Vitamin U fit into all this? It should be emphasized that there has not been any scientific research testing whether Vitamin U supplementation can prevent NTDs. The studies have simply not been done. However, there is some genetic evidence that suggests that Vitamin U may play a role in correct neural tube formation.
- Vitamin U supplies methyl groups to mammals via its reaction with homocysteine to form methionine catalyzed by the enzyme BHMT2. This is very similar to that of betaine, though whether Vitamin U plays this role in embryonic tissue has not been investigated.
- Vitamin U is abundant in vegetables, the benefits of which have been long known in preventing neural tube defects. While the presence of folate is most likely the primary factor, it is possible that some of the benefits conferred by eating vegetables are due to the provision of methyl groups from Vitamin U.
- A preconception diet rich in methionine reduces the prevalence of neural tube defects. Vitamin U is essentially methionine with an extra methyl group. One molecule of Vitamin U actually supplies two molecules of methionine, one being the newly-methylated homocysteine, the other being the demethylated Vitamin U.
- Studies have shown that methylation in the embryo is supplied by methyl groups from both the folate cycle and betaine. If we assume that methionine synthase and BHMT1 contribute to embryonic methylation, then Vitamin U is also likely to make a contribution. It is logical that the benefits of green vegetables in preventing neurological abnormalities is due to the combined effects of folate, betaine and Vitamin U, with the emphasis on folate.
Summary
- Folate is absolutely necessary at some level to provide the embryo nucleotides during the first weeks following conception. It cannot be replaced by other molecules.
- Folate requirements may be lowered as long as adequate levels of methyl groups are provided by methionine and betaine from the methionine cycle.
- Though its role in fetal development has not been investigated, it is likely that Vitamin U has a similar role to that of methionine and betaine, and would be of greater importance for people whose diet is low in protein and fat such as vegans.
Further Reading

